Top 6 Best Barrier Packaging Options for Medical Applications
Medical
December 7, 2022Reading time: 2 minutes
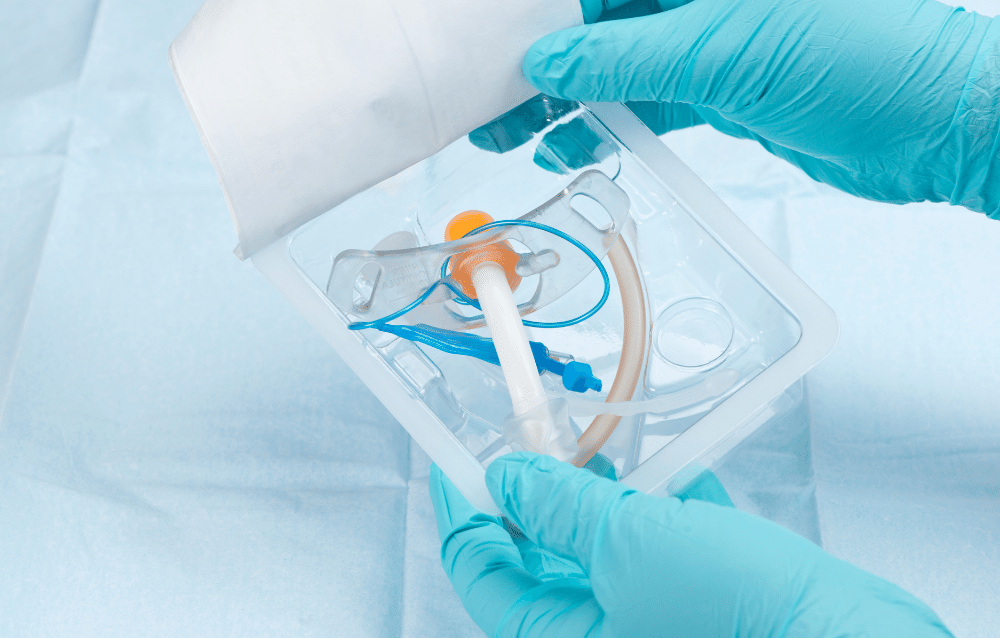
The U.S. Centers for Disease Control and Prevention (CDC) partially attribute advancements in barrier performance with a reported 5% decline in the operating room standardized infection ratio (SIR).* This is encouraging news for medical packaging producers, but healthcare-associated infections (HAIs) remain a concern — and focus area — for the healthcare industry.
Barrier packaging plays a role in helping manage HAI risk. Maximizing the impact begins with understanding how materials selection and package formats protect medical application sterility.
Sterile barrier packaging for healthcare
Sterile barrier packaging — also referred to as a barrier package system — is a primary package that houses and protects medical applications. The overarching goal is ensuring medical devices remain sterile, so the packaging must withstand aggressive cycles of one of three sterilization methods:
- Ethylene Oxide gas: a chemical agent that kills microorganisms by interfering with protein metabolism and cell reproduction
- Gamma radiation: a byproduct of disintegrated radioactive decay Cobalt 60 that sterilize disposable medical devices (syringes, needles, etc.) over an extended time frame of generally 10-20 hours
- Electron beam (E-beam): a process that transmits a high-energy beam of free electrons to penetrate and sterilize short production runs of low-density medical devices
To maintain a sterile packaging environment and also ensure easy, aseptic device removal from the package at the point of use, the method and barrier packaging materials must align. Working with an expert healthcare packaging partner is essential to match choices in foils, polyester, polyethylene, or DuPont™ Tyvek® solutions to specific needs.
Barrier and physical protection for medical devices
Likewise, due consideration must be given to device protection. The priority for barrier packaging is maintaining sterilization, but transportation, general handling, and storage can put protection at risk.
Barrier packaging for healthcare applications is structured in six general formats that offer varying degrees of device and seal protection:
-
Pouch: A low-cost solution for low-volume commodity products, a tear-open or peelable pouch is a versatile packaging option that provides little to no physical protection for devices.
-
Bag: Like pouches, tear-open bags serve as a cost-effective solution for drapes, gowns, kits, and bulk items such as Tyvek® garments. Physical protection is minimal.
-
Forming films: Forming films provide packaging with malleability and movement without sacrificing barrier protection. Forming films offer excellent sterilization and physical protection for medical devices such as prosthetics.
-
Laminations: Layers of films offer layers of protection — and that means a custom combination of defense mechanisms for specific devices to ensure quality performance throughout the life cycle.
-
Die cut lids: Die cut lids provide easy-open, aseptic point of use solutions. Cut to the shape of its adjacent tray and sealed to it with easy-peel technologies, die cut lids offer easy access to a device without disturbing its orientation, and assurance that no particulates are introduced into the healthcare environment.
-
Thermoformed trays: Thermoforming is an excellent choice in sterile and protective barrier packaging for healthcare applications. Thermoformed trays offer cavities formed to the exact device for snug nesting and better impact resistance and excellent physical protection. Transparent films offer excellent visibility and, when paired with appropriate lidding materials, the sterilization options are among the best in class for barrier protection.
From uses to materials and formats, the number of factors to consider when developing barrier packaging for medical products can be daunting. Expert and experienced healthcare packaging providers such as Amcor are invaluable partners in helping you determine the solution for your application, as is the advice you’ll find in The Insider’s Guide to Sterile Barrier Packaging.

SOURCE
*National Healthcare Safety Network/CDC, Surgical Site Infection Event, January 2022
DuPont™ and Tyvek® are trademarks or registered trademarks of E. I. du Pont de Nemours and Company or its affiliates.
Product Development Manager
