Desiccant packaging Dessiflex® supports the delivery of COVID-19 tests
Innovation
May 12, 2021Reading time: 4 minutes
The COVID-19 pandemic created an unprecedented global health crisis. Millions have been affected as healthcare systems and professionals have faced newly uncovered challenges all around the world. Yet in response to these extraordinary and uncertain times, resilience, teamwork and entrepreneurship have come together to find innovative ways to care for, diagnose and better protect the world’s population. One area where this is still apparent is within the diagnostics industry, which has been propelled to the front line in the ongoing fight against the SARS-CoV-2 virus.
Amcor's patented Dessiflex® packaging technology supports the protection and usability of IVD and POC test devices by incorporating the desiccant into the packaging’s sealant layer, eliminating the need for additional moisture scavengers. By removing the need for additional desiccant materials, this solution also has sustainability benefits, the packaging's carbon footprint can lower by up to 32% versus a similar pack of the same size.
The COVID-19 pandemic created an unprecedented global health crisis. Millions have been affected as healthcare systems and professionals have faced newly uncovered challenges all around the world. Yet in response to these extraordinary and uncertain times, resilience, teamwork and entrepreneurship have come together to find innovative ways to care for, diagnose and better protect the world’s population. One area where this is still apparent is within the diagnostics industry, which has been propelled to the front line in the ongoing fight against the SARS-CoV-2 virus.
Amcor's patented Dessiflex® packaging technology supports the protection and usability of IVD and POC test devices by incorporating the desiccant into the packaging’s sealant layer, eliminating the need for additional moisture scavengers. By removing the need for additional desiccant materials, this solution also has sustainability benefits, the packaging's carbon footprint can lower by up to 32% versus a similar pack of the same size.
ARTICLE UPDATED ON 14/01/2022
Back in September 2018, even before COVID-19 emerged, EvaluateMedTech®, was predicting in their World preview 2018-2024 report, that the in-vitro diagnostics market was estimated to grow at 6.1% (CAGR) and would “remain the number one medical device segment for the foreseeable future.”
As the world now responds to the concurrent waves COVID-19 and its variants, the role of diagnostic testing continues to be fundamental. Early detection is crucial to monitor the spread of the disease and to ensure the effective use of isolation, and where necessary quarantine measures. While over 9 billion vaccines have already been given and vaccine programmes continue to be rolled out, diagnostic testing remains an essential tool to continue to identify and treat individuals as well as assess the effectiveness of vaccines.
Two critical diagnostic procedures are being implemented to help manage the ongoing health crisis and diagnose COVID-19 infections.
Firstly, PCR based molecular tests illustrate if there is an active SARS-CoV-2 infection by detecting the virus’s genetic material. This type of testing is generally considered the most accurate, however, it typically requires swab samples to be sent to a lab for analysis. Results can sometimes take days to receive, and molecular tests still cannot be used to identify past positive cases.
Secondly, antigen testing - which also looks for active COVID infection by detecting specific proteins from the virus - can often be performed at the “point-of-care” (POC). This means the turnaround of results can be much quicker, now even made available within a few minutes in many cases. This has been best used for those already experiencing symptoms or those who have been exposed to a known case, to confirm the presence of the disease. The strategy of high-volume testing and rapid results followed by appropriate treatment has been shown to help limit further spread of COVID-19.
Just as important to preventing spreads is the development of serology tests. These are used to detect virus-specific antibodies and establish whether a person has been previously infected with SARS-CoV-2. This helps further map the progress and scale of the disease, identifying possible blood plasma donors for future treatment.
In all cases testing will be essential in the management of COVID-19 waves; providing information on the prevalence of the infection, supporting individuals to get the right care and protecting others.
The challenges facing diagnostic testing on this scale
While the need for mass testing is clear, this does present new challenges for diagnostic device manufacturers who need to respond to any sudden increase and spikes in demand. This places significant pressure on manufacturing and supply chains.
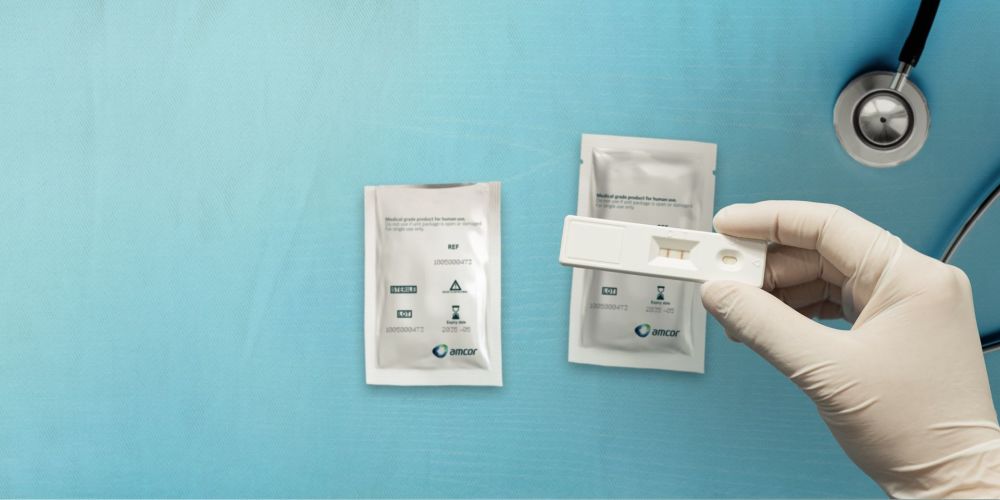
In-Vitro Diagnostics (IVD) are highly sensitive to their environment and need to be carefully protected to ensure their functionality and efficacy. Many require high barrier packaging and additional desiccants to control moisture. The packaging materials therefore play a critical role to ensure the safety and performance of the diagnostics device at the point of use.
Amcor's patented Dessiflex® packaging technology supports the protection and usability of IVD and POC test devices by incorporating the desiccant into the packaging’s sealant layer, eliminating the need for additional moisture scavengers. As our healthcare customers adapt and scale up supply of diagnostic test kits, Dessiflex® technology means we can provide a fully integrated, user-friendly packaging solution.
As our healthcare customers adapt and scale up supply of diagnostic test kits, Dessiflex® technology means we can provide a fully integrated, user friendly packaging solution.
Simplifying the packaging process helps to reduce packaging material inventories and streamline manufacturing. By integrating the desiccant into the film, this innovation effectively eliminates a whole step of the production process, reducing complexity at all stages of the supply chain. Dessiflex® technology also has enhanced sealing capabilities allowing for faster packaging speeds.
The built-in stability of the Dessiflex® technology protects diagnostic tests from moisture, even after the filling process, where residual humidity can persist within the packaging, extending the product's shelf life.
In addition to the manufacturing efficiencies, our customers have witnessed benefits for the end user as well. Additional desiccant sachets, alongside the device itself, can affect usability and present a safety risk. Using Dessiflex eliminates the risk of accidental ingestion or contamination. There are also sustainability benefits too. By successfully removing the need for additional desiccant materials, this solution can lower the packaging's carbon footprint by up to 32% versus a similar pack of the same size.
Overall, the combined impact of these benefits facilitates a more cost-efficient process for healthcare companies as well as a packaging that is safer and easier to handle.
Dessiflex® is a proven packaging technology and ready to scale with market demand
Amcor is perfectly placed to help fast-track validation procedures, increasing the speed to market for our customers.
During validation or for niche testing systems we can provide scalable orders of custom pouches that can be increased as customers build up orders in this dynamic market.
We also have the flexibility to keep pace with increased demand and grow with our customers. As demand for diagnostic testing increases, Amcor can provide roll stock materials to help meet the growth in demand for diagnostic devices.
Dessiflex® is a proven technology, used for years across a wide range of products within the healthcare industry to protect and ensure the integrity of moisture-sensitive devices and pharmaceuticals.
Click here to download our factsheet on Dessiflex for diagnostics.
We outline all the benefits of Dessiflex as well as provide detailed illustrations with comparison tables and data to help you to understand how you can better ensure the integrity of your devices and pharmaceuticals.
Find out more about our full heathcare packaging range here.
Would you like to stay up to date on the latest medical packaging trends? Sign up to receive regular information, invitations to webinars about sustainability, regulations and other current packaging topics
Contact us to get in touch with our team and learn more about packaging solutions for diagnostics.
Healthcare Europe Team
Email: afemeahcmarketing@amcor.com
Related Insights
Meeting demand for syringe packaging to support COVID-19 vaccination
April 29, 2021
Many vaccines have now become available with vaccination programmes in place around the globe to combat COVID-19 and its variants. However, this means there are logistical challenges that need to be addressed when rolling-out programmes on such a large scale.
Many vaccines have now become available with vaccination programmes in place around the globe to combat COVID-19 and its variants. However, this means there are logistical challenges that need to be addressed when rolling-out programmes on such a large scale.
Dessiflex®: innovative medical packaging for diagnostic applications
October 21, 2021
Discover how Dessiflex technology is shifting diagnostics packaging in a fast-changing market, how this perfect medical packaging protects sensitive IVD tests whilst extending shelf life, increasing sustainability and reducing costs.
Discover how Dessiflex technology is shifting diagnostics packaging in a fast-changing market, how this perfect medical packaging protects sensitive IVD tests whilst extending shelf life, increasing sustainability and reducing costs.
