Blister vs. Bottle: Is There a Clear Winner in Pharma Packaging?
Pharma
December 28, 2022Reading time: 3 minutes
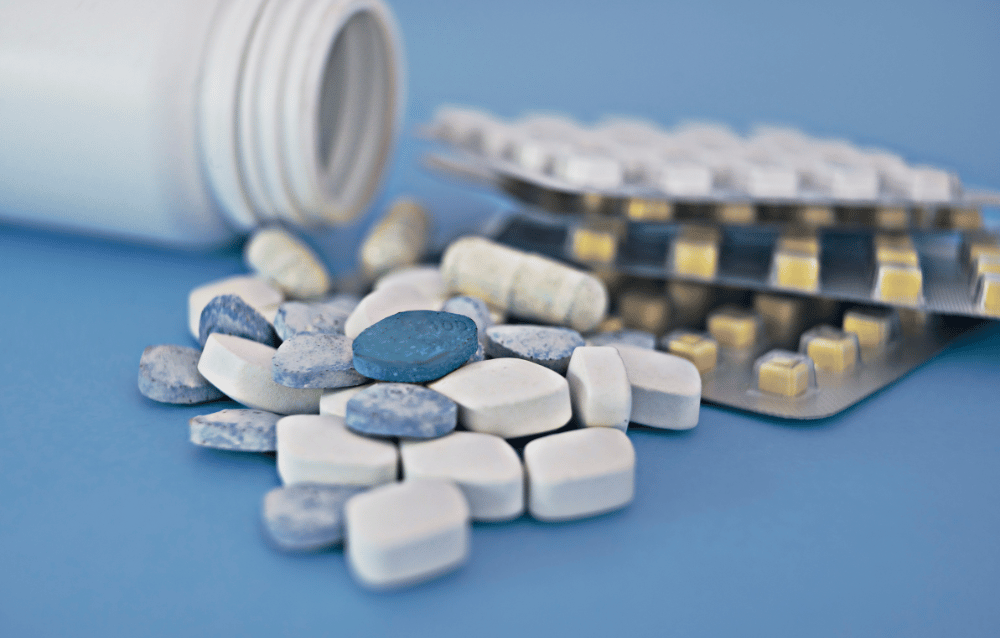
In the United States, pharmaceutical oral solid dosages are dispensed in bottles about 80% of the time. European countries, on the other hand, prefer using blister packaging by roughly the same percentage.1
The global dichotomy in types of pharma packaging isn’t meant to suggest one solution is better than the other; it’s simply an observation that fuels the blister vs. bottle debate. Whatever the packaging choice, there are commonalities in performance: patient safety and convenience, drug stability and efficacy, contamination prevention, etc.
However, there is projected 5-year growth of the blister packaging markets in the U.S. and the EU (5.60% and 6.45%, respectively1). Trends such as sustainability and the proliferation of ecommerce are driving packaging in the pharmaceutical industry toward blister, but that doesn’t preclude the use of bottles.
The Legacy of Bottles
A solution since the 19th century, glass and plastic bottles are ubiquitous in pharmaceutical packaging. The protection, portability, size and labeling versatility, and general cost-effectiveness still make bottles viable packaging for the industry.
Market factors and forces will ultimately determine the future use of bottles in the United States. What could influence some decisions, however, is how blister packaging is coming to the forefront as a preferred format for child safety and anti-counterfeit protection, better long term drug stability and integrity, and improved administration of medications by patients.2
Blister Redefines Types of Pharma Packaging
The construction of blister packs directly addresses and corrects the concerns often raised about bottles, and also provides additional benefits:
-
Contamination: Blister packaging is efficient in mitigating contamination risk. Proven hermetic seals prevent exposure to contamination until the time of use and maximize shelf life by preventing ingress of moisture.
-
Dosing: Since capsules are individually dispensed users can more readily track their dosing in order to maintain compliance with their prescriptions. Requesting refills is also easier for users as they can quickly assess their prescription or OTC medication levels.
-
Child-Resistant and Senior-Friendly Openings: The types of packaging material in pharma blisters provide opportunities for innovation. A traditional bottle cap offers an option to prevent child poisoning, but at the same time may also limit senior accessibility. Blisters, on the other hand, can be designed to accommodate myriad solutions. Amcor’s PushSmart™, for example, is an effective and convenient child-resistant and senior-friendly lidding available. The innovative design incorporates micro-perforated “Release Zones” for push-through access, enabling seniors to easily release capsules.
-
Right Sizing: Medications typically rattle in prescription bottles, increasing the potential for damage to individual pills — which could interfere with efficacy or per-pill dosage accuracy. Blister packs provide snug protection of individual capsules, using only the materials necessary to keep capsules intact and prevent waste.
-
Sustainability: Historically, a move from a pill bottle to a blister pack required moving from a readily recycled HDPE, PET, or PP container to a blister package using PVC and foil which are not recyclable. That is a tough move to make when companies are focused on improving the sustainability of their products and packaging. However, that has changed with the breakthrough AmSky™ Blister System. AmSky is a recyclable HPDE-based blister solution that provides pharmaceutical and consumer health manufacturers with a low carbon footprint, recycle-ready sustainable package that supports the circular economy. It allows pharmaceutical and nutraceutical companies to move from bottles to blisters while maintaining recyclability.
Pharmaceutical manufacturers can and often need to strike a balance between using bottles and blister packs. The needs of the medications, market channels, and end users will define and ultimately determine packaging decisions.
As blister packaging becomes more sophisticated, it is increasing opportunities for greater performance in prescription and retail applications. Find out more about the advantages in opting for blister solutions in our guide, Exploring Pharmaceutical Blister Packaging Opening Features.

SOURCES
1Accupack Engineering, Blister Packaging vs Bottles for Pharmaceutical Products - Accupack, Undated
2ProMach, Pharmaworks, The USA and a Shift from Bottles to Blisters: What the Rest of the World Already Knows, July 1, 2022